The Bentall procedure is a cornerstone of complex cardiac surgery, demanding precision, adaptability, and deep expertise from the entire heart team—especially the perfusionist. This surgery, which replaces the aortic valve, aortic root, and ascending aorta while reimplanting the coronary arteries, is a high-stakes operation often described as the “Super Bowl” for perfusionists due to its complexity and critical need for flawless cardiopulmonary bypass (CPB) management. Below, I’ll expand on the provided outline with updated insights, detailed strategies, and modern considerations to help perfusionists dominate CPB management for the Bentall procedure.
What is the Bentall Procedure?
The Bentall procedure, first described by Hugh Bentall and Antony De Bono in 1968, is a composite graft replacement surgery addressing pathologies of the aortic root, aortic valve, and ascending aorta. It involves:
- Replacement of the aortic valve: Typically with a mechanical (e.g., On-X, St. Jude) or bioprosthetic valve (e.g., Edwards Inspiris, Medtronic Freestyle).
- Replacement of the aortic root and ascending aorta: Using a Dacron graft to restore structural integrity.
- Reimplantation of the coronary arteries: Coronary ostia are detached as “buttons” and reattached to the graft to maintain myocardial perfusion.
The procedure has evolved with modifications like the modified Bentall (using a Valsalva graft to mimic the sinuses of Valsalva) and bio-Bentall (using tissue valves for younger patients or those avoiding anticoagulation). It remains the gold standard for complex aortic root pathologies.
Common Indications
- Ascending aortic aneurysms: Especially those >5.5 cm or rapidly expanding (>0.5 cm/year).
- Aortic root dilation with aortic valve disease: Including aortic regurgitation or stenosis.
- Marfan syndrome and connective tissue disorders: E.g., Loeys-Dietz syndrome, Ehlers-Danlos syndrome.
- Stanford Type A aortic dissection: Acute or chronic, requiring urgent intervention.
- Bicuspid aortic valve (BAV) with aneurysm: Increasingly common due to improved imaging and screening.
- Infective endocarditis: With root abscess or extensive destruction.
- Failed prior aortic repairs: Including valve-sparing procedures or redo scenarios.
Surgical Anatomy and Key Considerations
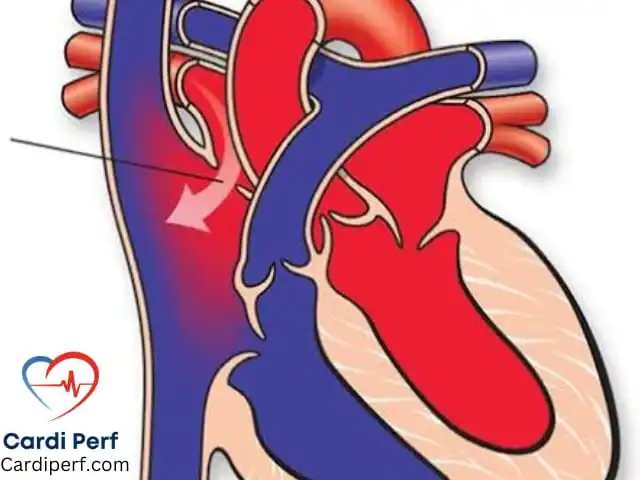
The Bentall procedure requires intimate knowledge of the aortic root’s anatomy:
- Aortic root and annulus: The functional unit where the valve cusps, sinuses of Valsalva, and sinotubular junction converge.
- Coronary ostia: Precise detachment and reimplantation are critical to avoid kinking or malperfusion.
- Left ventricular outflow tract (LVOT): Must be preserved to ensure proper valve function post-repair.
- Proximity to critical structures: Mitral valve, pulmonary artery, and right ventricle are at risk during dissection.
Perfusionist’s Role in Anatomy:
- Timing cardioplegia delivery to protect the myocardium during root dissection.
- Monitoring coronary perfusion post-reimplantation to detect ischemia (e.g., ST-segment changes on ECG).
- Ensuring adequate flow during CPB weaning to support the newly reconstructed root.
Role of the Perfusionist in Bentall Surgery
The perfusionist is the linchpin of the operating room (OR) during a Bentall procedure, orchestrating CPB to maintain systemic perfusion, protect the myocardium, and adapt to intraoperative challenges. Key responsibilities include:
- Regulating cerebral perfusion: Especially during aortic arch manipulation or circulatory arrest.
- Ensuring myocardial protection: Via precise cardioplegia delivery.
- Managing hemodynamic shifts: Rapid changes due to cross-clamping, unclamping, or bleeding.
- Adapting to emergencies: E.g., acute dissection requiring alternative cannulation.
- Coordinating with the team: Communicating with surgeons and anesthesiologists for seamless transitions (e.g., cross-clamp timing, rewarming).
The perfusionists also leverage advanced technologies like real-time cerebral oximetry (e.g., INVOS or FORE-SIGHT systems) and inline blood gas analyzers to optimize outcomes.
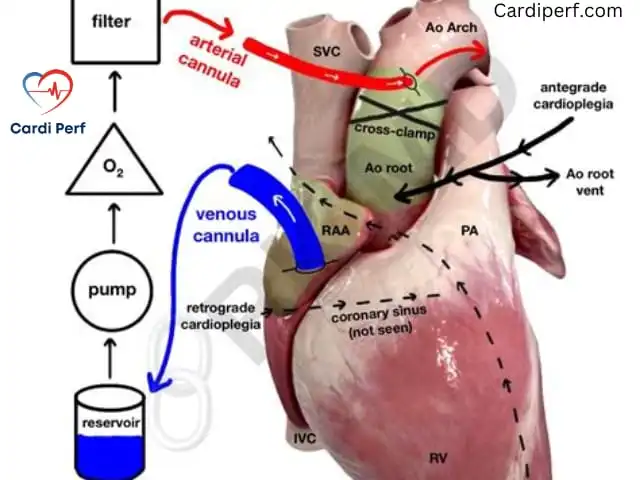
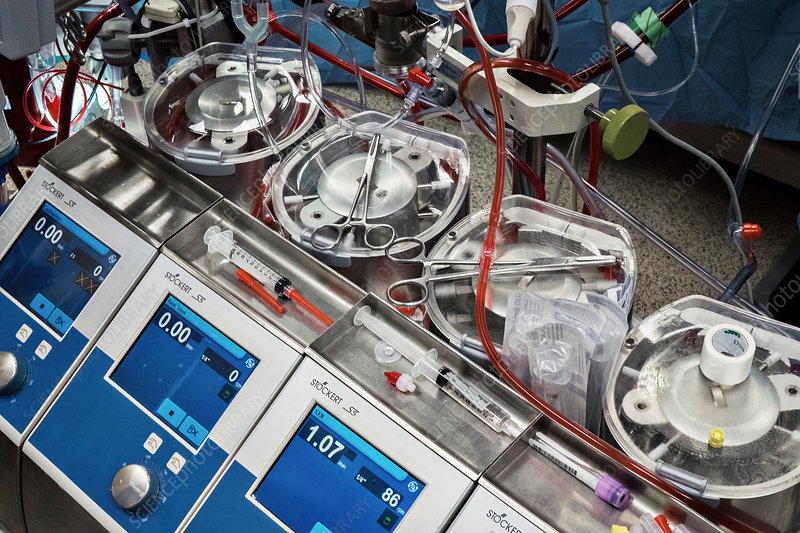
Cardiopulmonary Bypass (CPB) Setup
A robust CPB setup is the foundation of a successful Bentall procedure. The circuit must be versatile to handle prolonged bypass times, high blood loss, and potential circulatory arrest.
Essentials
- Closed CPB circuit: Heparin-coated tubing reduces inflammatory response and thrombus formation.
- Cardioplegia system: Modular setup for antegrade, retrograde, and direct ostial delivery. Modern systems (e.g., Quest MPS3) allow precise control of cardioplegia temperature and pressure.
- Hemoconcentrator and cell saver: Integrated to manage hemodilution and conserve blood.
- Venous reservoir: High-capacity to buffer volume shifts during dissection or bleeding.
- Vacuum-assisted venous drainage (VAVD): Enhances venous return, especially in redo cases or with bicaval cannulation.
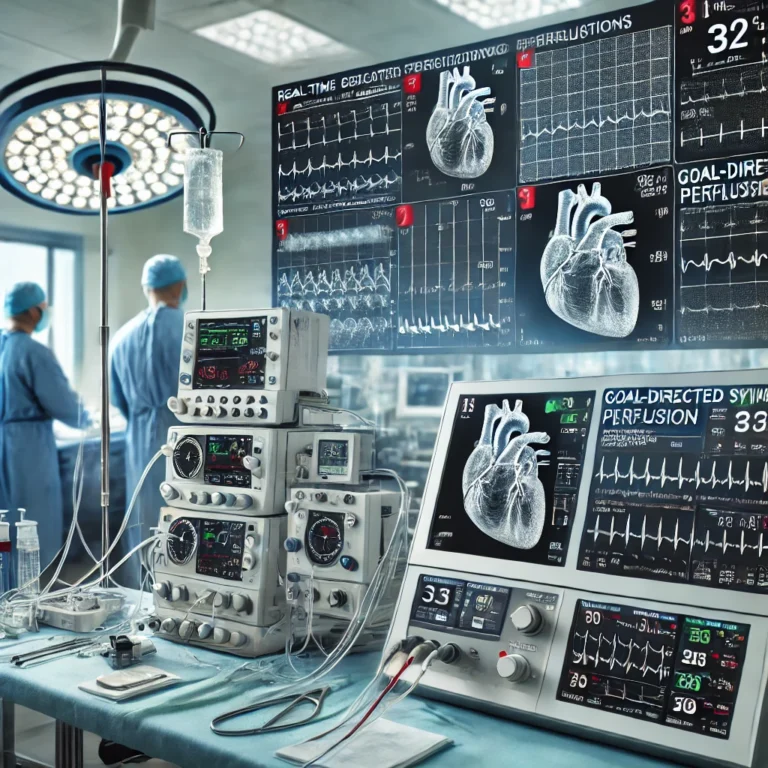
- Monitoring suite:
- Cerebral oximetry (near-infrared spectroscopy, NIRS) for real-time brain oxygenation trends.
- Inline arterial and venous blood gas monitoring (e.g., Terumo CDI 550).
- Pressure transducers for arterial, venous, and cardioplegia lines.
- Transesophageal echocardiography (TEE) integration for real-time cardiac assessment.
Hemodilution and Prime Strategy
- Prime composition:
- Crystalloid: Balanced electrolyte solution (e.g., Plasma-Lyte) with sodium bicarbonate (50–100 mEq) to buffer pH.
- Mannitol: 12.5–25 g to reduce edema and promote diuresis.
- Heparin: 3000–5000 IU to coat the circuit and prevent clotting.
- Packed red blood cells (PRBCs): Added if preoperative hematocrit (HCT) <35% or body surface area (BSA) is small (<1.8 m²).
- Electrolytes: Calcium (0.5–1 g), magnesium (1–2 g), and potassium (10–20 mEq) to stabilize membranes and prevent arrhythmias.
- Target HCT: 22–28% to balance oxygen delivery and viscosity, adjusted for patient-specific factors (e.g., renal function, baseline anemia).
- Prime volume: 1000–1500 mL, minimized to reduce hemodilution in smaller patients.
Cannulation Strategies
- Arterial cannulation:
- Ascending aorta: Preferred for stable aneurysms without dissection.
- Femoral artery: Used in acute dissections or calcified aortas.
- Axillary artery: Increasingly popular for antegrade cerebral perfusion (ACP) in arch-involved cases.
- Venous cannulation:
- Two-stage cannula: Standard for most cases, placed in the right atrium.
- Bicaval cannulation: Preferred for right heart isolation, especially if opening the right atrium or ventricle is needed.
- Alternative approaches: In redo surgeries or dissections, peripheral cannulation (femoral or axillary) may be established before sternotomy to mitigate bleeding risks.
Monitoring Arsenal
- Activated clotting time (ACT): Maintain >480 seconds before and during CPB to ensure anticoagulation.
- Hemodynamic parameters:
- Mean arterial pressure (MAP): 60–80 mmHg, with >70 mmHg for cerebral perfusion.
- Central venous pressure (CVP): 2–6 mmHg to guide volume status.
- Venous oxygen saturation (SvO₂): >65% to confirm adequate tissue perfusion.
- Temperature probes:
- Nasopharyngeal: Reflects cerebral temperature.
- Bladder: Indicates core body temperature.
- Esophageal: Monitors cardioplegia and myocardial temperature.
- Cerebral oximetry: Tracks regional oxygen saturation (rSO₂) to detect cerebral ischemia, with alarms for >20% drop from baseline.
- Inline monitors: Real-time HCT, pH, pCO₂, and lactate levels to guide acid-base management.
Myocardial Protection: Cardioplegia Game Plan
Myocardial protection is paramount during the Bentall procedure, as prolonged cross-clamp times (often 90–150 minutes) increase the risk of ischemic injury.
Cardioplegia Strategy
- Induction:
- Antegrade: Cold blood cardioplegia (4:1 or 8:1 blood:crystalloid ratio) delivered via the aortic root or coronary ostia (800–1000 mL at 8–12°C).
- Pressure: 80–100 mmHg to ensure adequate coronary perfusion without endothelial damage.
- Maintenance:
- Repeated every 20–25 minutes via antegrade (root) or retrograde (coronary sinus) routes.
- Direct ostial cardioplegia is critical after the aorta is opened to ensure delivery to both coronary arteries.
- Hot-shot (terminal warm cardioplegia):
- 500 mL warm (35–37°C) blood cardioplegia just before cross-clamp release to restore metabolic substrates and reduce reperfusion injury.
- Monitoring:
- Cardioplegia line pressure: 80–100 mmHg.
- Myocardial temperature: Maintain 8–12°C to minimize metabolic demand.
- TEE: Assess for ventricular distension or inadequate arrest.
Advanced Techniques
- Del Nido cardioplegia: Increasingly used for single-dose or less frequent dosing (every 60–90 minutes), reducing interruptions during complex root work.
- Custodiol (HTK solution): Considered in select cases for prolonged single-dose protection, especially in redo or dissection scenarios.
- Microplegia systems: Deliver concentrated cardioplegia with minimal crystalloid, reducing hemodilution.

Temperature Management
Temperature control is critical for organ protection, particularly for the brain and heart.
Options
- Normothermic CPB (34–36°C):
- Rare, used in select cases (e.g., valve-sparing root procedures) to avoid hypothermia-related coagulopathy.
- Requires meticulous myocardial protection due to higher metabolic demand.
- Moderate hypothermia (28–32°C):
- Standard for most Bentall procedures, balancing organ protection and surgical time.
- Reduces cerebral and systemic oxygen demand.
- Deep hypothermic circulatory arrest (DHCA, 18–20°C):
- Used in cases involving the aortic arch or acute dissections.
- Adjuncts: Topical ice packs around the head, EEG for burst suppression, and NIRS for cerebral oxygenation.
- Antegrade cerebral perfusion (ACP) via axillary or innominate artery cannulation is often combined to extend safe arrest times (up to 30–40 minutes).
Rewarming
- Rate: Gradual, no faster than 1°C every 3–5 minutes to prevent cerebral hyperthermia or protein denaturation.
- Gradient: Maintain <10°C difference between arterial inflow and venous return to avoid gas bubble formation or tissue injury.
- Target: Core temperature of 36–37°C before weaning from CPB.
Volume Management and Blood Conservation
Bentall procedures are notorious for significant blood loss due to extensive dissection and suture lines (e.g., coronary reimplantation, graft anastomoses).
Strategies
- Hemoconcentration: Use a hemofilter in the CPB circuit to remove excess fluid and maintain HCT.
- Cell saver: Process shed blood to return autologous red cells, reducing transfusion needs.
- Antifibrinolytics:
- Tranexamic acid (TXA): 10 mg/kg bolus + 1–2 mg/kg/h infusion.
- Epsilon-aminocaproic acid (EACA): 5 g bolus + 1 g/h infusion.
- Point-of-care coagulation testing:
- Rotational thromboelastometry (ROTEM) or thromboelastography (TEG) to guide transfusion of platelets, fresh frozen plasma (FFP), or cryoprecipitate.
- Ultrafiltration:
- Conventional ultrafiltration (CUF): During CPB to remove inflammatory mediators.
- Modified ultrafiltration (MUF): Post-CPB to concentrate blood and improve hemodynamics.
Transfusion Triggers (2025 Guidelines)
- Hemoglobin <7–8 g/dL during CPB.
- Platelets <100,000/µL if bleeding persists.
- Fibrinogen <150 mg/dL or abnormal ROTEM/TEG.
Critical Parameters to Monitor
| Parameter | Target Range | Notes |
|---|---|---|
| Flow Rate | 2.4–2.6 L/min/m² | Adjust based on BSA and metabolic demand. |
| MAP | 60–80 mmHg | >70 mmHg for cerebral perfusion in high-risk patients. |
| CVP | 2–6 mmHg | Guides volume management; avoid overfilling. |
| Venous Saturation | >65% | Indicates adequate tissue perfusion. |
| ACT | >480s | Ensure anticoagulation; recheck every 30 minutes. |
| HCT | 22–28% | Balance oxygen delivery and viscosity. |
| Lactate | <2 mmol/L | Rising lactate suggests hypoperfusion. |
Special Scenarios in Bentall Surgeries
Acute Aortic Dissection
- Cannulation: Femoral or axillary artery due to unstable aortic root.
- Cerebral protection: Initiate ACP during DHCA to minimize embolic risk.
- Monitoring: Watch for malperfusion syndromes (e.g., renal, mesenteric) via lactate and TEE.
Reoperation (Redo Sternotomy)
- Risks: Adhesions increase bleeding risk; inadvertent entry into the aorta or right ventricle is possible.
- Strategy: Establish femoral or axillary cannulation before sternotomy. Have rapid infusion systems (e.g., Belmont Rapid Infuser) ready.
- Backup: ECMO circuit on standby for failure to wean from CPB.
Coronary Button Malperfusion
- Signs: ST-segment elevation, ventricular arrhythmias, or poor myocardial recovery post-cross-clamp.
- Action: Immediate ostial cardioplegia or revision of coronary anastomoses. Prepare for intra-aortic balloon pump (IABP) or ECMO if recovery fails.
FAQs: Bentall Procedure and Perfusionist Role
1. What is the Bentall procedure, and why is it considered complex?
Answer: The Bentall procedure is a composite graft surgery that replaces the aortic valve, aortic root, and ascending aorta, with reimplantation of the coronary arteries. It’s complex due to prolonged cross-clamp times (90–150 minutes), significant blood loss, and the need for precise myocardial and cerebral protection. Perfusionists must manage CPB, cardioplegia, and potential emergencies like acute aortic dissection, making it a high-stakes operation.
2. What is the perfusionist’s role in the Bentall procedure?
Answer: The perfusionist is the “pulse of the OR,” managing CPB to maintain systemic perfusion, delivering cardioplegia for myocardial protection, and monitoring cerebral oxygenation and hemodynamics. They adapt to rapid changes (e.g., bleeding, coronary malperfusion) and coordinate with surgeons and anesthesiologists to ensure smooth transitions, such as cross-clamp release or weaning from bypass.
3. What are the key components of the CPB circuit for a Bentall procedure?
Answer: A Bentall CPB circuit includes:
- Heparin-coated tubing (closed system) to reduce inflammation.
- Modular cardioplegia system for antegrade, retrograde, and ostial delivery.
- Hemoconcentrator and cell saver for blood conservation.
- High-capacity venous reservoir with vacuum-assisted venous drainage (VAVD).
- Inline monitors for blood gas, hematocrit, and cerebral oximetry (e.g., NIRS).
4. How is cardioplegia administered during the Bentall procedure?
Answer: Cardioplegia is critical for myocardial protection:
- Induction: Cold blood cardioplegia (4:1 or 8:1 ratio) delivered antegrade (800–1000 mL, 8–12°C).
- Maintenance: Every 20–25 minutes via antegrade or retrograde routes, or direct ostial delivery after aortic opening.
- Hot-shot: Warm cardioplegia (500 mL, 35–37°C) before cross-clamp release.
- Modern option: Del Nido cardioplegia for single-dose or less frequent administration.
5. What temperature management strategies are used in Bentall surgery?
Answer: Temperature control protects organs:
- Moderate hypothermia (28–32°C): Standard for most cases, balancing organ protection and surgical time.
- Deep hypothermic circulatory arrest (DHCA, 18–20°C): Used for arch involvement or dissections, with antegrade cerebral perfusion (ACP) and NIRS monitoring.
- Rewarming: Gradual (1°C every 3–5 minutes), keeping arterial-venous gradient <10°C.
6. How does a perfusionist manage blood loss in Bentall surgery?
Answer: Blood conservation is key due to high-volume loss:
- Hemoconcentration: Use a hemofilter to remove excess fluid.
- Cell saver: Recycle shed blood to reduce transfusions.
- Antifibrinolytics: Tranexamic acid (10 mg/kg bolus + 1–2 mg/kg/h) or epsilon-aminocaproic acid.
- Coagulation monitoring: ROTEM/TEG to guide platelet or plasma transfusion.
- Ultrafiltration: Conventional (CUF) during CPB or modified (MUF) post-CPB.
7. What are the critical parameters to monitor during CPB for Bentall?
Answer:
- Flow rate: 2.4–2.6 L/min/m², adjusted for BSA.
- Mean arterial pressure (MAP): 60–80 mmHg (>70 mmHg for cerebral perfusion).
- Central venous pressure (CVP): 2–6 mmHg.
- Venous oxygen saturation (SvO₂): >65%.
- Activated clotting time (ACT): >480 seconds.
- Hematocrit (HCT): 22–28%.
- Lactate: <2 mmol/L to detect hypoperfusion.
8. How do perfusionists handle acute aortic dissection during a Bentall procedure?
Answer: Acute dissection requires:
- Alternative cannulation: Femoral or axillary artery due to unstable aortic root.
- Cerebral protection: Antegrade cerebral perfusion (ACP) during DHCA to minimize embolic risk.
- Monitoring: Watch for malperfusion (e.g., renal, mesenteric) via lactate levels and TEE.
- Backup: ECMO circuit on standby for failure to wean from CPB.
9. What challenges arise in redo Bentall surgeries?
Answer: Redo sternotomies involve:
- Adhesion risks: Increased bleeding from dense scar tissue.
- Cannulation strategy: Femoral or axillary cannulation before sternotomy to mitigate bleeding risks.
- Monitoring: Enhanced vigilance for right ventricular or aortic injury.
- Backup: Rapid infusion systems (e.g., Belmont) and ECMO readiness for hemodynamic instability.
10. How can perfusionists prepare for coronary button malperfusion?
Answer: Coronary malperfusion is a critical complication:
- Signs: ST-segment elevation, ventricular arrhythmias, or poor myocardial recovery.
- Action: Immediate ostial cardioplegia or revision of coronary anastomoses.
- Monitoring: Real-time ECG and TEE for ischemia detection.
- Backup: Prepare for intra-aortic balloon pump (IABP) or ECMO if the heart fails to recover.
11. What’s the difference between antegrade and retrograde cardioplegia in Bentall surgery?
Answer:
- Antegrade: Delivered via the aortic root or coronary ostia; faster induction but requires intact coronary arteries.
- Retrograde: Via the coronary sinus; ideal when the aorta is opened or coronaries are diseased, but may miss right ventricular protection.
- Hybrid approach: Often used in Bentall cases, alternating antegrade and retrograde for complete myocardial coverage.
12. Why is cerebral oximetry important in Bentall procedures?
Answer: Cerebral oximetry (NIRS) monitors regional oxygen saturation (rSO₂) to detect cerebral ischemia, especially during DHCA or arch manipulation. A >20% drop from baseline signals hypoperfusion, prompting adjustments in flow, MAP, or ACP. It’s critical for neuroprotection in complex aortic cases.
13. What are the latest advancements in perfusion for Bentall surgery ?
Answer:
- Del Nido cardioplegia: Single-dose or less frequent dosing for prolonged protection.
- Microplegia systems: Minimize crystalloid volume to reduce hemodilution.
- Advanced monitoring: Inline blood gas analyzers (e.g., Terumo CDI 550) and real-time ROTEM/TEG.
- Smart circuits: Automated CPB systems with AI-driven flow adjustments (e.g., Terumo’s latest platforms).
- Custodiol (HTK): Used in select cases for single-dose myocardial protection.
14. How can perfusionists prepare for emergencies during Bentall surgery?
Answer:
- Pre-op checklist: Verify circuit integrity, prime composition, and backup systems (e.g., ECMO).
- Scenario planning: Prepare for dissection (femoral cannulation), bleeding (cell saver), or malperfusion (ostial cardioplegia).
- Team communication: Coordinate with surgeons and anesthesiologists for rapid response to ST changes or hemodynamic shifts.
- Training: Regular simulation for redo sternotomy or DHCA scenarios.
15. Where can I find a perfusion checklist for Bentall surgery?
Answer: Download a comprehensive “Bentall Perfusion Checklist” PDF from Cardiperf.com, covering CPB setup, prime strategy, cardioplegia protocols, and monitoring parameters. It’s designed for perfusionists to streamline preparation and ensure no step is missed.