In the delicate realm of cardiac surgery, meticulous fluid management is paramount. Today, we’re diving deep into the critical processes of ultrafiltration cardiac surgery, specifically contrasting Conventional Ultrafiltration (CUF) and Modified Ultrafiltration (MUF). This article will illuminate the core principles of each, exploring the procedural nuances, patient benefits, and crucial differences between CUF vs MUF. From explaining the standard applications of CUF during cardiopulmonary bypass to highlighting the advanced techniques of MUF post-bypass, we’ll decode how these methods impact patient outcomes and best practices.
Table of Contents
What is Conventional Ultrafiltration (CUF) in Cardiac Surgery?
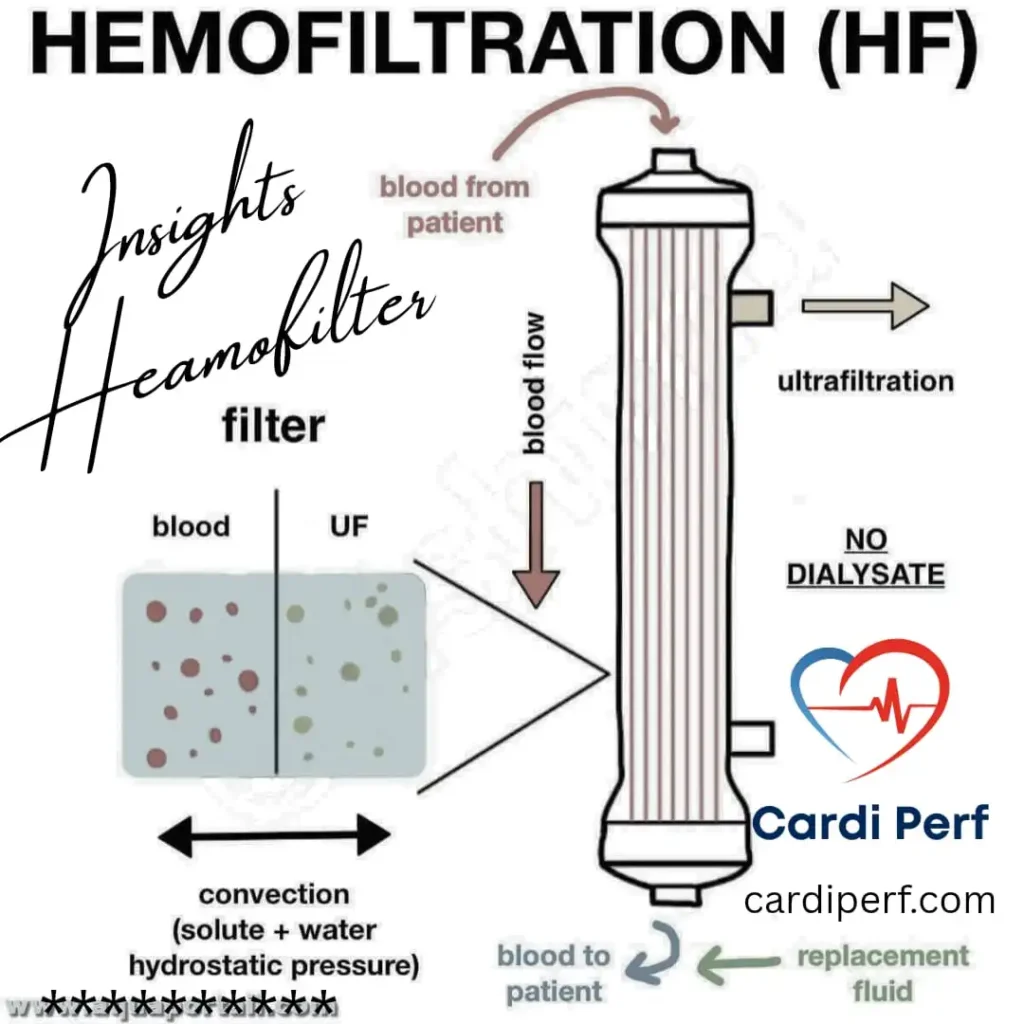
Conventional Ultrafiltration (CUF) is a technique employed during cardiopulmonary bypass (CPB) to remove excess fluid from the patient’s blood. During CPB, the patient’s blood is diverted through a heart-lung machine, which oxygenates and circulates it. CUF is integrated into this process, where a specialized filter, or ultrafilter, is used to remove water and solutes from the blood plasma.
The typical setup involves connecting the ultrafilter to the CPB circuit. As blood passes through, hydrostatic pressure forces water and small molecules (like electrolytes) across the filter membrane, leaving behind larger molecules (like proteins and blood cells). This process helps to concentrate the blood, reducing hemodilution and minimizing fluid overload.
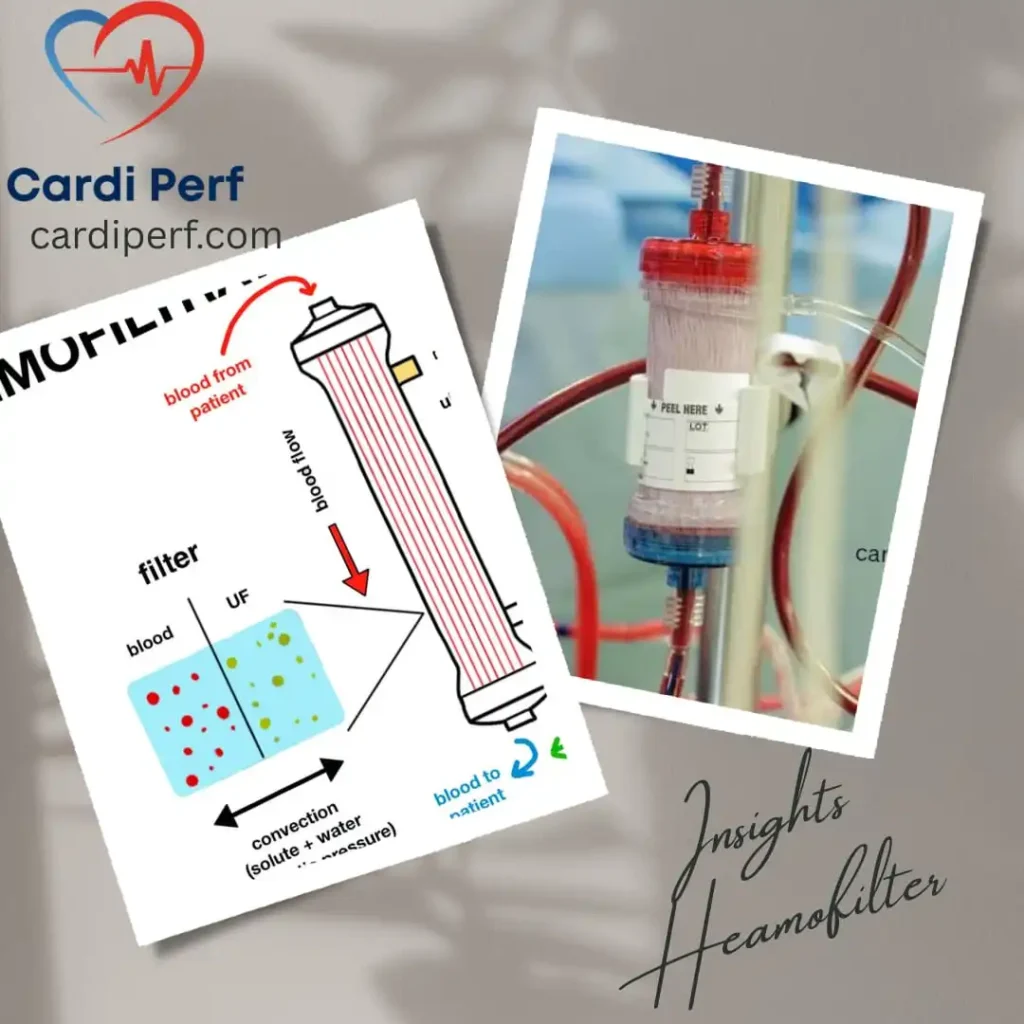
The primary goals of CUF are to maintain optimal blood volume, improve hematocrit levels, and prevent complications related to fluid excess, such as pulmonary edema. It is a standard practice in many cardiac surgeries, particularly those involving prolonged CPB.
What is Modified Ultrafiltration (MUF)in Cardiac Surgery?
Modified Ultrafiltration (MUF) is a technique performed after the completion of cardiopulmonary bypass. Unlike CUF, which occurs during CPB, MUF is applied once the patient’s heart has resumed its function. MUF involves recirculating the residual blood from the CPB circuit through an ultrafilter, removing excess fluid and inflammatory mediators.
CUF in Cardiac Surgery: Definition & Mechanism
Conventional Ultrafiltration (CUF) is a continuous process integrated directly into the cardiopulmonary bypass (CPB) circuit. Its primary role is to actively counteract the hemodilution from the circuit prime and administered fluids during the procedure, serving as a method for continuous ultrafiltration cardiopulmonary bypass management.
Mechanism & Perfusionist-Level Detail:
- Circuit Setup: The hemofilter is connected as a shunt from a high-pressure point (typically the arterial line, post-oxygenator) to a low-pressure point (the venous reservoir). This pressure gradient provides the hydrostatic driving force.
- Driving Force: Main CPB circuit pressure. The ultrafiltration rate is controlled by a clamp or regulator on the ultrafiltrate collection line.
- Key Parameters:
- Flows: The flow through the CUF circuit is passive and determined by circuit pressure, typically representing 100-500 mL/min of the total flow.
- Ultrafiltration Rate: Managed to achieve a target net negative fluid balance (e.g., 1-2 L for an adult), always with vigilant monitoring of venous reservoir level to prevent air entrainment.
- Priming: The CUF circuit is primed as an integral part of the overall CPB circuit. No additional priming volume is required.
- Expected Hematocrit (Hct) Changes: The effect is gradual. A practical benchmark is an expected rise of 1-3 percentage points in the circuit Hct for every liter of ultrafiltrate removed. This is highly dependent on starting Hct and total volume ultrafiltered.
MUF in Cardiac Surgery: Definition & Mechanism
Modified Ultrafiltration (MUF) is a post-bypass intervention performed after the patient is weaned from CPB but while still cannulated. It is a powerful technique for rapidly reversing the residual effects of CPB by concentrating the patient’s blood volume and removing inflammatory mediators.
Mechanism & Perfusionist-Level Detail:
- Circuit Setup (Arteriovenous Method): After CPB is concluded, the circuit is reconfigured. The arterial cannula becomes the blood inlet, a dedicated roller pump draws blood through the hemofilter, and the concentrated blood is returned to the patient via the venous cannula.
- Driving Force: A dedicated roller pump on the MUF circuit.
- Key Parameters:
- Flows: Typically 100-200 mL/min for adults and 50-100 mL/min for pediatric patients. Must be titrated to the patient’s hemodynamic response (MAP, CVP).
- Duration: Usually 10-20 minutes, or until a target ultrafiltrate volume (e.g., 20-40 mL/kg) or a desired final hematocrit is achieved.
- Priming: The MUF circuit requires a separate prime. This prime should be recirculated to waste or discarded before connection to the patient to avoid an initial crystalloid bolus.
- Expected Hematocrit (Hct) Changes: MUF produces a rapid and significant correction. It is common to see a total Hct rise of 5-10 percentage points (e.g., from 24% to 30-34%), as it concentrates the entire volume of blood processed.
CUF vs MUF: Immediate Physiological Effects
This table contrasts the direct physiological impacts of each technique, which guides their strategic application.
| Physiological Parameter | Conventional Ultrafiltration (CUF) | Modified Ultrafiltration (MUF) |
|---|---|---|
| Hemodilution | Gradual correction during CPB. Manages the process of dilution. | Rapid, significant reversal after CPB. Corrects the outcome of dilution. |
| Inflammatory Mediators | Limited removal efficacy; inflammatory response is ongoing during CPB. | Highly effective removal; processes the peak inflammatory load present at the end of CPB. |
| Hemodynamic Status | Effect is isolated to the CPB circuit volume. Minimal direct impact on patient BP. | Direct and rapid effect on patient BP and preload. Requires active titration and communication with anesthesia. |
| Blood Product Exposure | Can reduce need by maintaining a higher Hct in the circuit. | More effectively reduces need by concentrating the patient’s final circulating volume. |
| Core Temperature | No direct effect. | Can contribute to patient rewarming if the blood returning from the hemofilter is warmed. |
CUF vs MUF: Clinical Outcomes & Evidence
The clinical evidence solidifies the theoretical advantages, particularly for MUF.
- Pediatric Cardiac Surgery: The evidence here is overwhelming. A meta-analysis in the Journal of Cardiac Surgery concluded that MUF in children, compared to CUF or no UF, leads to:
- Significantly higher post-operative hematocrit.
- Improved systolic blood pressure and cardiac index.
- Reduced ventilator hours and shorter ICU length of stay.
- Lower levels of inflammatory markers (IL-6, TNF-α).
- Adult Cardiac Surgery: The benefits in adults are most pronounced in high-risk patients. Studies, including those in the Annals of Thoracic Surgery, have shown that MUF is associated with:
- Reduced incidence of Acute Kidney Injury (AKI) due to improved fluid balance and reduced inflammatory insult.
- Lower inotrope scores and improved hemodynamic stability post-bypass.
- Trends towards reduced transfusion requirements and shorter time to extubation.
Conclusion: While CUF is a valuable intraoperative tool for volume control, MUF provides a superior post-bypass “rescue” strategy with a stronger evidence base for improving concrete clinical outcomes, especially in vulnerable populations.
Perfusionist Practical Protocol: How to Implement MUF/CUF
A clear, step-by-step modified ultrafiltration protocol is essential for safety and efficacy.
CUF Protocol:
- Pre-CPB: Integrate the hemofilter into the CPB circuit (arterial line to venous reservoir). Ensure the ultrafiltrate line is clamped.
- Initiation: After stable CPB flow is achieved and the patient is cooled, open the ultrafiltrate collection line. Adjust the clamp to achieve the desired negative fluid balance, constantly ensuring the venous reservoir level remains safe.
- Monitoring: Continuously track ultrafiltrate output on the fluid balance sheet. Monitor circuit Hct periodically to gauge efficacy.
- Termination: Discontinue CUF during the weaning phase of CPB.
MUF Protocol:
- Pre-Bypass: Prime the MUF circuit separately and have it ready. Ensure the roller pump is calibrated.
- Post-Bypass (Post-Protamine): Confirm hemodynamic stability with the surgeon and anesthesiologist. Connect the arterial and venous lines to the MUF circuit.
- Initiation: Start the MUF roller pump at a low flow (e.g., 50 mL/min). Gradually increase to the target flow (100-200 mL/min for adults) while the anesthesiologist monitors blood pressure and central venous pressure.
- Execution: Maintain target flow for a predetermined duration (10-20 mins) or until a goal volume is ultrafiltered. The team should be prepared to administer volume or adjust vasoactive drugs as needed.
- Termination: Gradually decrease the MUF pump flow to zero. Clamp the arterial and venous MUF lines. Disconnect the circuit. The patient’s blood volume is now concentrated and optimized.
Key Take-aways for Perfusion Teams
- CUF is for Maintenance; MUF is for Optimization: Use CUF for continuous fluid management during CPB. Use MUF as a powerful tool to “reset” the patient’s physiology after CPB.
- MUF is a Team Sport: Its success is 100% dependent on constant, clear communication with the surgeon and anesthesiologist. Announce flow changes and report volume removed.
- Advocate for the Patient: Based on the evidence, proactively suggest MUF for all pediatric cases and complex or high-risk adult cases (renal impairment, poor EF, long cross-clamp time).
- Data Drives Decisions: Know your expected Hct changes. A poor response may indicate issues with the filter or circuit.
- Safety First: With MUF, the patient is the reservoir. Vigilance for air embolism and circuit integrity is paramount.
Goals of CUF in Cardiac Ultrafiltration
The process involves connecting the ultrafilter to the arterial and venous lines of the CPB circuit after the patient is weaned off the machine. This allows the residual blood in the circuit to be processed. MUF is particularly beneficial for removing inflammatory mediators that accumulate during CPB, which can contribute to postoperative complications.
The rationale behind MUF is to further optimize fluid balance, improve hemodynamic stability, and reduce the systemic inflammatory response. It is especially advantageous for pediatric patients, high-risk adults, and those with compromised renal function.

Key Differences Between CUF and MUF in Ultrafiltration Cardiac Surgery: A Comparative Analysis
Timing Differences: CUF vs. MUF in Cardiac Ultrafiltration
- Timing: CUF is performed during CPB, while MUF is done after CPB. This difference in timing significantly impacts the physiological effects and clinical applications.
- Technique: CUF is integrated into the CPB circuit, focusing on fluid removal during the procedure. MUF, on the other hand, involves processing residual blood after CPB, targeting both fluid and inflammatory mediators.
- Goals: CUF primarily aims to manage fluid volume and improve blood concentration during CPB. MUF focuses on optimizing fluid balance and reducing the inflammatory response post-CPB.
Patient Selection for Ultrafiltration Cardiac Surgery Methods
- Patient Selection: CUF is a routine practice in many cardiac surgeries. MUF is often preferred for pediatric patients, high-risk adults, and those with renal dysfunction.
- Outcomes: Both techniques improve patient outcomes, but MUF is associated with reduced inflammatory response, improved hemodynamic stability, and fewer postoperative complications.
| Feature | Conventional Ultrafiltration (CUF) | Modified Ultrafiltration (MUF) |
| Timing | During Cardiopulmonary Bypass (CPB) | After Cardiopulmonary Bypass (CPB) |
| Technique | Integrated into CPB Circuit | Recirculation of Residual CPB Blood |
| Primary Goal | Fluid Volume Management | Fluid Balance & Inflammation Reduction |
| Patient Selection | General Cardiac Surgery Patients | Pediatric, High-Risk, Renal Dysfunction |
| Outcomes | Improved Blood Concentration | Reduced Inflammation, Improved Hemodynamics |
Benefits and Advantages of MUF Over CUF
MUF offers several advantages over CUF, particularly in reducing the systemic inflammatory response. CPB triggers the release of inflammatory mediators, which can lead to postoperative complications. MUF effectively removes these mediators, minimizing their adverse effects.
MUF also improves hemodynamic stability by optimizing fluid balance and reducing the need for blood transfusions. By concentrating the residual blood in the CPB circuit, MUF minimizes hemodilution and improves oxygen-carrying capacity.
Clinical studies have demonstrated that MUF reduces the incidence of postoperative complications, such as acute kidney injury (AKI) and pulmonary edema. It is particularly beneficial in pediatric cardiac surgery, where fluid management is critical due to the smaller blood volume and immature renal function.
Potential Limitations and Considerations
While MUF offers significant benefits, it also has potential limitations. Patient selection is crucial, as MUF may not be suitable for all patients. Contraindications include severe coagulopathy and unstable hemodynamic conditions.
The learning curve for MUF can be steep, requiring specialized training and expertise. Proper monitoring and assessment are essential to ensure optimal outcomes.
Ongoing research continues to refine ultrafiltration techniques and identify new applications. Advancements in filter technology and monitoring systems are improving the safety and efficacy of MUF.
Clinical Applications and Best Practices
Both CUF and MUF have broad clinical applications in cardiac surgery. CUF is a standard practice during CPB, while MUF is increasingly used in pediatric and high-risk adult patients.
Individualized patient management is essential. The choice between CUF and MUF should be based on patient-specific factors, such as age, comorbidities, and the complexity of the surgical procedure.
Monitoring fluid balance, electrolyte levels, and hemodynamic parameters is crucial during and after ultrafiltration. Regular assessment of renal function is also essential to detect and manage potential complications.
Clinical guidelines and recommendations from professional organizations provide valuable guidance on the appropriate use of ultrafiltration techniques.
Conclusion (Summary & Call to Action)
In summary, both CUF and MUF play vital roles in optimizing fluid management during and after cardiac surgery. CUF is a standard practice during CPB, while MUF offers significant advantages in reducing inflammation and improving hemodynamic stability post-CPB.
The benefits of MUF are particularly evident in pediatric and high-risk adult patients. As research continues to advance, ultrafiltration techniques will become even more refined, improving patient outcomes and minimizing postoperative complications.
Stay informed about the latest advancements in ultrafiltration by subscribing to our updates. For more information or to discuss your specific needs, please contact us.
We hope this comprehensive overview has clarified the differences between CUF and MUF, providing valuable insights into these critical techniques in modern cardiac surgery.
Frequently Asked Questions (FAQs)
1. What is the primary difference between Conventional Ultrafiltration (CUF) and Modified Ultrafiltration (MUF)?
- Answer: CUF is performed during cardiopulmonary bypass (CPB) to remove excess fluid from the blood circulating through the heart-lung machine. MUF is performed after CPB, using the residual blood in the CPB circuit to remove additional fluid and inflammatory mediators.
2. Who are the ideal candidates for Modified Ultrafiltration (MUF)?
- Answer: MUF is particularly beneficial for pediatric patients, high-risk adults, and patients with compromised renal function. It helps minimize postoperative complications and improve overall outcomes in these populations.
3. Does Modified Ultrafiltration (MUF) reduce the risk of acute kidney injury (AKI) after cardiac surgery?
- Answer: Yes, studies have shown that MUF can significantly reduce the incidence of AKI by optimizing fluid balance and minimizing the inflammatory response that can contribute to kidney damage.
4. What are the benefits of removing inflammatory mediators during Modified Ultrafiltration (MUF)?
- Answer: Removing inflammatory mediators helps reduce the systemic inflammatory response, which can lead to complications like pulmonary edema, hemodynamic instability, and prolonged recovery.
5. How is the volume of fluid removed during ultrafiltration monitored?
- Answer: The volume of fluid removed is carefully monitored using specialized equipment and techniques. This includes measuring the weight of the ultrafiltrate and monitoring the patient’s hemodynamic parameters.
6. Is Modified Ultrafiltration (MUF) a standard practice in all cardiac surgeries?
- Answer: While MUF is increasingly used, it is not a standard practice in all cardiac surgeries. The decision to use MUF depends on patient-specific factors and the complexity of the procedure.
7. What are the potential risks associated with Modified Ultrafiltration (MUF)?
- Answer: Potential risks include complications related to blood recirculation, such as clotting or air embolism. Proper monitoring and adherence to best practices can minimize these risks.
8. Can Modified Ultrafiltration (MUF) reduce the need for blood transfusions?
- Answer: Yes, MUF can help concentrate the patient’s blood, reducing hemodilution and minimizing the need for blood transfusions.
9. How does Modified Ultrafiltration (MUF) improve hemodynamic stability?
- Answer: MUF improves hemodynamic stability by optimizing fluid balance, reducing the inflammatory response, and improving blood concentration, which enhances oxygen-carrying capacity.
10. Where can surgeons learn more about advanced techniques in Modified Ultrafiltration (MUF)?
- Answer: Surgeons can learn more through specialized training programs, clinical workshops, and by staying updated on the latest research and publications in cardiac surgery. Professional organizations also offer valuable resources and guidelines.
11.When is MUF used in paediatric vs adult bypass?
Answer: The use of Modified Ultrafiltration (MUF) is far more standardized and frequent in pediatric cardiac surgery compared to adult surgery, reflecting the different physiological challenges in these populations.
In Paediatric Bypass:
MUF is considered a standard of care in many pediatric heart centers worldwide. It is used proactively in the vast majority of cases involving cardiopulmonary bypass, especially:
- Neonates and Infants: This group is most vulnerable to the deleterious effects of hemodilution due to their extremely small blood volume. Even a small amount of circuit prime can lead to severe hemodilution.
- Complex Cyanotic Procedures: Patients with single-ventricle physiology (e.g., Norwood, Glenn, Fontan procedures) benefit greatly from the improved hemodynamics and reduced inflammatory load.
- All Cases with Prolonged CPB Time: MUF is crucial for mitigating the cumulative inflammatory and fluid overload associated with long, complex repairs.
In Adult Bypass:
MUF is used more selectively in adults, reserved for high-risk patients where the benefits clearly outweigh the added procedural time. Its use is indicated in:
- Patients with Pre-existing Renal Dysfunction: MUF helps offload fluid and inflammatory mediators, reducing the risk of post-operative acute kidney injury (AKI).
- Cases with Significant Hemodilution: When the calculated transfusion threshold is breached (e.g., Hct < 20-22%) at the end of CPB, MUF can rapidly concentrate the blood and avoid homologous blood transfusions.
- Patients with Poor Cardiac Function: Those with low ejection fraction struggle to manage fluid shifts post-bypass. MUF provides a “dryer” and more concentrated blood volume, easing the workload on the heart and improving hemodynamic stability.
- Complex Re-do Surgeries or Long Cross-Clamp Times: These cases generate a significant inflammatory response, which MUF effectively attenuates.
12.What are typical hematocrit rise values after MUF?
Modified Ultrafiltration produces a rapid and significant increase in hematocrit (Hct). The typical rise is a key performance indicator for the procedure.
The expected hematocrit rise after MUF is typically 5 to 10 percentage points.
Examples:
- A pediatric patient weaned from bypass with a Hct of 24% would be expected to have a final Hct between 29% and 34% after a standard MUF run.
- An adult patient starting at 22% could realistically reach 27% to 32%.
Important Factors Influencing the Rise:
This value is not absolute and depends on several factors:
- Starting Hematocrit: Patients with a lower pre-MUF Hct will generally see a larger absolute increase.
- Volume Ultrafiltered: The total volume of fluid removed is the primary driver. A common protocol is to target 20-40 mL per kg of patient body weight.
- Patient’s Blood Volume: The same volume of ultrafiltrate will cause a greater Hct rise in a smaller patient (e.g., a 5 kg infant) than in a larger adult.
- MUF Circuit Priming: If the circuit prime is not adequately cleared before connection, the initial hemodilution will attenuate the final Hct result.
This predictable and substantial rise is a major benefit of MUF vs CUF, as it directly reduces the need for packed red blood cell transfusions and improves oxygen-carrying capacity at a critical point in the patient’s recovery.